What is a Patent?
As agencies of the U.S. Government, NIH owns the rights to any patent to a discovery made by any NIH employee or personnel working at a NIH facility, or from a discovery that involves use of a NIH facility or use of government equipment. In general, NIH inventors must assign their rights to inventions they develop in their official duty, to the NIH. However, each NIH inventor is recognized as an inventor on any issued patent, and upon assignment of his/her rights to any invention to the NIH, such inventor will receive a share of any royalties obtained through licensing the invention, whether it is patented or not.
NIH employees and staff are required to report inventions in response to Federal laws and encouraged to help facilitate the commercial development of such discoveries, through further research and collaboration and then through patenting and licensing, in order to generate products and services that will benefit the public health.
Reportable inventions include:
- Compounds (or other compositions of matter)
- Devices; Methods of use
- Unique biological materials with commercial applications such as transgenic mice and cell lines
A patentable invention must be:
- non-obvious to those trained in that area of research
- novel
- have a practical use
Timely reporting of discoveries is critical, because patent protection may be lost if an invention is publicly disclosed prior to filing a patent application.
A public disclosure may include:
- Talks, presentations, posters
- Publications, including titles and abstracts posted on websites
- Internet postings
- Graduate student theses, job interviews
- Discussions with non-NIH personnel without a Confidential Disclosure Agreement (CDA) in place
The NIH Patent Process
The general NCI patent process is described below.
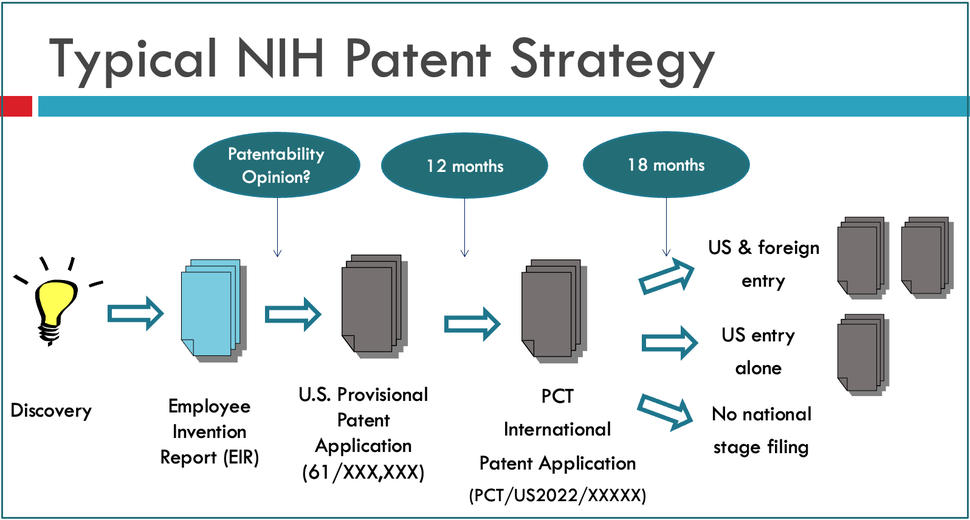
The first step in the NIH patent process is to report a discovery through filling an EIR form and submitting it to TTC for handling and processing. An invention review will be scheduled to conduct a comprehensive evaluation of the reported EIR and make a decision on patent filing strategy. Should the NIH Institute/Center (IC) agree to file a patent application, TTC will send the EIR to a law firm for patent application preparation and filing with USPTO. At different stages of patenting, the inventors will work closely with the patent attorney at the contract law firm on the preparation and prosecution of the patent application to seek the issuance of a patent.
It is NIH general practice that a U.S. provisional patent application will be filed first, to establish the priority date of invention. The provisional patent protects the intellectual property for 365 days, allowing the inventor and the TTC to discuss the invention publicly and to further develop the invention and supporting data, and add new details and information, in further support of the drafted claims of the patent application. Prior to the one year anniversary, as the provisional patent application is expiring, a checkpoint will be reached for TTC to make a recommendation and the lead NIH institute to make a determination whether or not to continue the application, and file a preliminary filing under the Patent Cooperation Treaty (PCT international patent application), usually designating all contracting countries/regions. If prosecution of a PCT patent application is continued, the patent application will enter the National Stage of foreign filing at thirty months from the priority date set by the provisional patent application. At this decision point, NIH will designate which countries it wishes to continue to seek patent protection in.
A U.S. patent application must be filed prior to any public disclosure of an invention to preserve international patent rights and must be filed within one year of the official publication date or public use to preserve U.S. patent rights. After a patent application is filed with USPTO, patent attorneys at a contract law firm will work on patent prosecution seeking the issuance of patent. The general patent process may take averagely 3~5 years. At different stages of this process, there are several checkpoints where TTC staff will obtain invention update from NCI inventors for new rounds of review and analysis to justify the continued filing/prosecution for pursuing a patent. Patent applications may be abandoned due to variable reasons or considerations.
Once a U.S. patent application is filed, TTC will update its preliminary marketability and patentability analysis and prior to 12-month filing deadline, a recommendation regarding international filing is made. In general, where international filing is possible and one can reasonably anticipate commercial interest, TTC recommends at least a preliminary filing under the Patent Cooperation Treaty (PCT), 12 months after the U.S. filing date, to preserve international rights for an additional 18 months at modest cost. Upon agency determination to exercise international patent rights, the contract attorney arranges for international patent prosecution. In parallel with the filing of a patent application, TTC reviews the invention and its commercial potential, develops a licensing approach, and identifies potential companies to commercialize the invention. This is coordinated by the TTC Specialist and TTC Licensing and Patenting Managers under whose case dockets the invention lies, and is a collaborative process requiring input from the inventors, and the TDC. After a patent application is filed, a formal advertising campaign for potential licensees and co-developers is developed to promote the technology to companies and other collaborative partners.
TTC Staff reviews all new inventions submitted, and facilitates the whole patent process. If you need any assistance in this process, please contact the Technology Transfer Center.
NIH Patent Policy
The Public Health Service (PHS) may determine that it is necessary to seek patent protection on its inventions, when doing so would facilitate the commercial development of products or services that will benefit the public health, or when a patent will advance another PHS objective.
The decision whether or not to file a patent application will also be informed by PHS policies, as appropriate, including, for example, the NIH Policies on Research Tools and Genomic Inventions. Therefore, multiple factors will be weighed, in consideration of making a strategic decision on whether or not to file. Such factors can include the patentability of an invention, the stage of invention development, the commercialization potential of a product generated from an invention, and the mission and programmatic goals the NIH institution, as well as the research interest toward further invention development by the lead inventive NIH laboratory.
Below are listed some common reasons why an invention might not get patented:
- Past public disclosures by the inventor or other scientists in the field may limit or destroy the patentability of an invention. This situation can be avoided by submitting your EIR to the TTC well in advance of any contemplated public disclosures;
- The invention is in an early stage, such that a patent application would not result in the issuance of a patent by the USPTO (e.g. proof-of-concept is not well supported). You will be encouraged to contact the TTC when additional information is available for re-evaluation of the technology. TTC staff will assist inventors in such situations and will advise as to when an EIR should be re-submitted;
- The claims of the patent are too narrow to attract licensing by a commercial entity. In this instance, it is generally not recommended to file a patent application; the inventor will be given feedback on further research goals that would allow them to further develop the inventive concept and is encouraged to submit an updated EIR at a later time.
- The Institute's Division Director decides against filing a patent application or subsequently decides to abandon a patent application. If this is the case, an inventor may request a waiver to obtain the rights to the invention. If you are interested in requesting a waiver, or in learning more about the implications of a securing a waiver, please contact TTC for more information;
- NIH Policy requires that certain inventions remain in the public domain (e.g. methods of performing surgical procedures) in order to ensure that they are freely and widely available. For instance, the NIH generally does not seek patent protection on the inventions which can be defined as “Research Tools”, although NCI has the authority to license non-patented biological materials and substances through a Biological Material License (BML), for which inventors can still receive royalties.
Research Tools, also called research resources or research materials, are biological or other materials that are:
- Primarily useful for research purposes, such as to the elucidation of disease mechanisms or to drug discovery;
- By definition, are finished products that often do not require further development time and development costs in order to be utilized; and
- Broadly enabling inventions, useful in developing multiple products in numerous disciplines, rather than a single project-specific or product-specific use.
Common examples of Research Tools include:
- antibodies
- expression plasmids and proteins derived from them
- cell receptors
- cell lines
- animal models (e.g., knockout mice)
- laboratory and drug-screening methods or protocols
- certain software