FDA Approval of First Immunotherapy for Recurrent Respiratory Papillomatosis
- Updated:
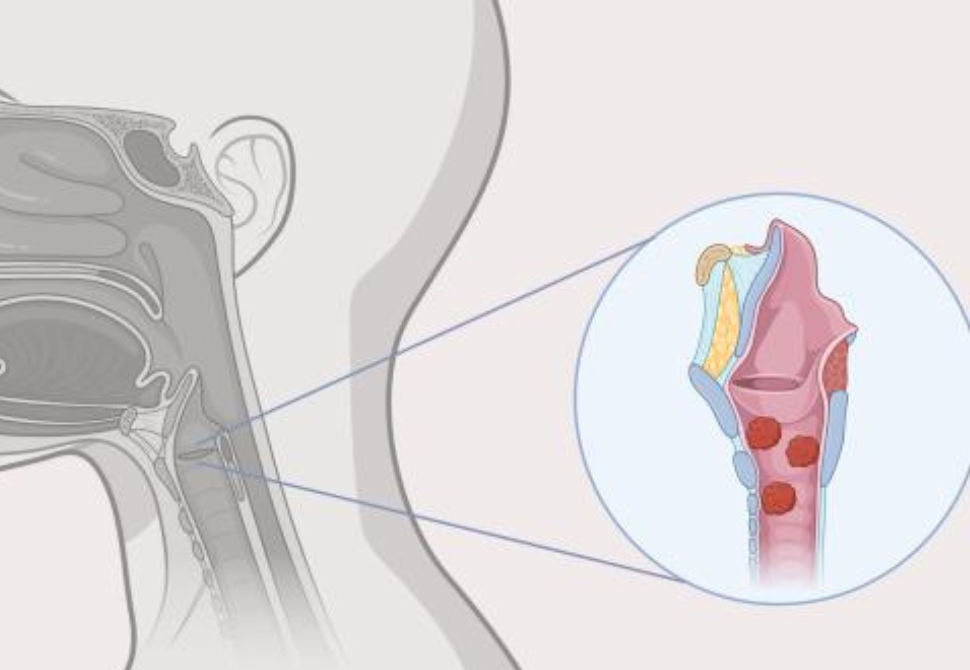
An illustration of papillomas in the throat, caused by recurrent respiratory papillomatosis (RRP). Newly approved Papzimeos is the first immunotherapy to provide a non-surgical treatment option for RRP.
Credit: Image created by Linda Wang with BioRender.
On August 14, 2025, the U.S. Food and Drug Adminstration granted full market approval for Papzimeos (zopapogene imadenovec-drba), a groundbreaking immunotherapy designed for adult patients with recurrent respiratory papillomatosis (RRP). With the approval, these patients have a new treatment for this rare and potentially life-threatening disease.
Papzimeos was developed through a partnership between researchers at NCI's Center for Cancer Research, part of the National Institutes of Health, and the biopharmaceutical company, Precigen. Under a Cooperative Research and Development Agreement (CRADA) facilitated by NCI's Technology Transfer Center, Scott Norberg, D.O., and Client Allen, M.D. led a pivotal, clinical study. The findings from the clinical trial were published January 17, 2025, in The Lancet Respiratory Medicine. The approval was completed under Priority Review and the product received both Orphan Drug designation and Breakthrough Therapy designation.
People with RRP develop small, wart-like growths (papillomas) in the upper and lower airways and lungs that can obstruct breathing and lead to respiratory complications. Patients require repeated surgeries to remove lesions as they grow back and spread. Until now, management of RRP primarily consisted of repeated surgeries, which do not address the root cause of the disease and can be associated with significant morbidity as well as significant patient and health system burden.
TTC's Michael Pollack, Ph.D., led the technology transfer effort supporting the collaboration between NCI and Precigen. Suna Gulay French, Ph.D. and Zehra Sherwani, J.D. managed a second CRADA with Precigen focused on correlation studies of specimens collected during the Phase 1/2 Papzimeos trial.