Webinar: Radiolabeled Sugars for Imaging of Fungal Infections

May 28, 2025 | 11:00 AM – 12:00 PM
The National Institutes of Health Clinical Center (NIHCC) is seeking research co-development partners and/or licensees for a newly developed fungal-specific imaging agent. Contact Joseph.Conrad@nih.gov to request a recording of our May 2025 webinar to learn about the technology and hear from NIH inventors, Dima A. Hammoud, MD, and Rolf Swenson, Ph.D..
About the Technology
The Problem:
Fungal infections remain a major health burden with very high mortality and morbidity in immunosuppressed cancer and transplant patients, and in some congenital immunodeficiencies. A recent report estimated global mortality from fungal disease to be greater than 1.6 million, which is similar to that of tuberculosis; and greater than 3-fold that of malaria. Yet, despite the magnitude of the problem, diagnosing a fungal infection in a patient is difficult because there are currently no clinically available fungal-specific imaging agents.
Standard of Care:
Currently, 18F-fluorodeoxglucose (FDG), a positron emission tomography (PET) radiotracer which is used to help diagnose cancer and neurological diseases, is being used to diagnose fungal and bacterial infections. However, FDG has a challenge of not being able to differentiate between a tumor, infection and inflammation. It also cannot differentiate between a variety of fungal pathogens or differentiate fungi from other pathogens such as bacteria.
Researchers investigated varying approaches to develop fungus-specific imaging including targeted antibodies, 99mTc-labeled MORF oligomers targeting fungal ribosomal RNA (rRNA), and radiolabeled siderophores. Many of these approaches are still in early-stage development, and need testing in humans, so a new fungal-specific ligand-based imaging agent is still needed.
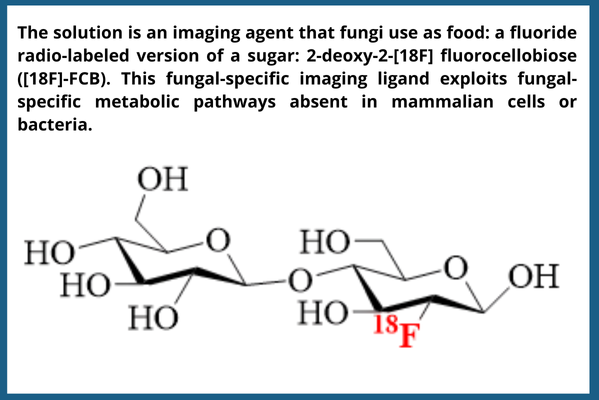
The Solution:
Researchers at the NIH Clinical Center developed a new fungal-specific imaging ligand that exploits fungal-specific metabolic pathways absent in mammalian cells or bacteria.
FCB:
- Shows specific uptake by pathogenic fungi, mostly molds (Aspergillus, mucor species) as it is hydrolyzed only by fungal beta-glucosidases and is not metabolized by bacterial or human enzymes.
- Demonstrates a high fungal infection imaging signal compared to normal tissues.
- Can be used to detect the presence of a fungus shortly after infection, thus providing early, specific diagnosis without the need for invasive procedures, such as biopsies, fine-needle aspirates or bronchoalveolar lavage.
- Can be used to monitor the infection in vivo and assess whether there is response to antifungal treatments in real time.
Competitive Advantages
- Rapid diagnosis of live fungal infections in any patient.
- Distinguish between fungal infection and other diseases such as tumors, inflammation or bacterial infections.
- Capacity for national and international distribution, via an easy-synthesis chemistry kit.
Commercial Applications:
- Fungal metabolism-based imaging agent, specific for pathogenic fungal molds versus current Standard of Care.
- Non-invasive patient imaging procedure providing rapid diagnosis and ability to monitor treatment.
- Easily synthesized from commercially available FDG.
Who should attend?
- Business development professionals
- Parties interested in scouting for technologies to diagnose fungal infections
- Biotech/pharma/academia researchers
- Investors and entrepreneurs
Why attend?
- Assess co-developing the technology
- Interact with the inventor, ask questions and provide feedback
- Learn how to partner with the NIH facilitated by the NCI Technology Transfer Center.
Learn more about the technology.
If you are interested in learning more about how to co-develop and/or license this technology, please contact Michael.Salgaller@nih.gov.