Technology ID
TAB-3969
Nandrolone 17 Beta-Carbonates
E-Numbers
E-181-2004-0
Lead Inventors
Blye, Richard
Co-Inventors
Kim, Hyun
Lee, Min
Applications
Therapeutics
Therapeutic Areas
Reproductive Health
Endocrinology
Development Stages
Clinical Phase I
Lead IC
NICHD
ICs
NICHD
The available options for male contraceptives are limited. Androgens are administered as part of hormone-based male contraception and have also been used in the treatment of hypogonadism and hormone replacement therapy HRT. Many synthetic androgens require an oil-based delivery vehicle and have limited durations of action.
This technology describes androgenic compounds and pharmaceutical compositions thereof for use in a number of diseases or conditions, most notably as a potential male contraceptive, and as an androgenic agent suppressing the release of hormones such as the luteinizing hormone. Additional potential therapeutic areas include hypogonadism, osteoporosis, and anemia.
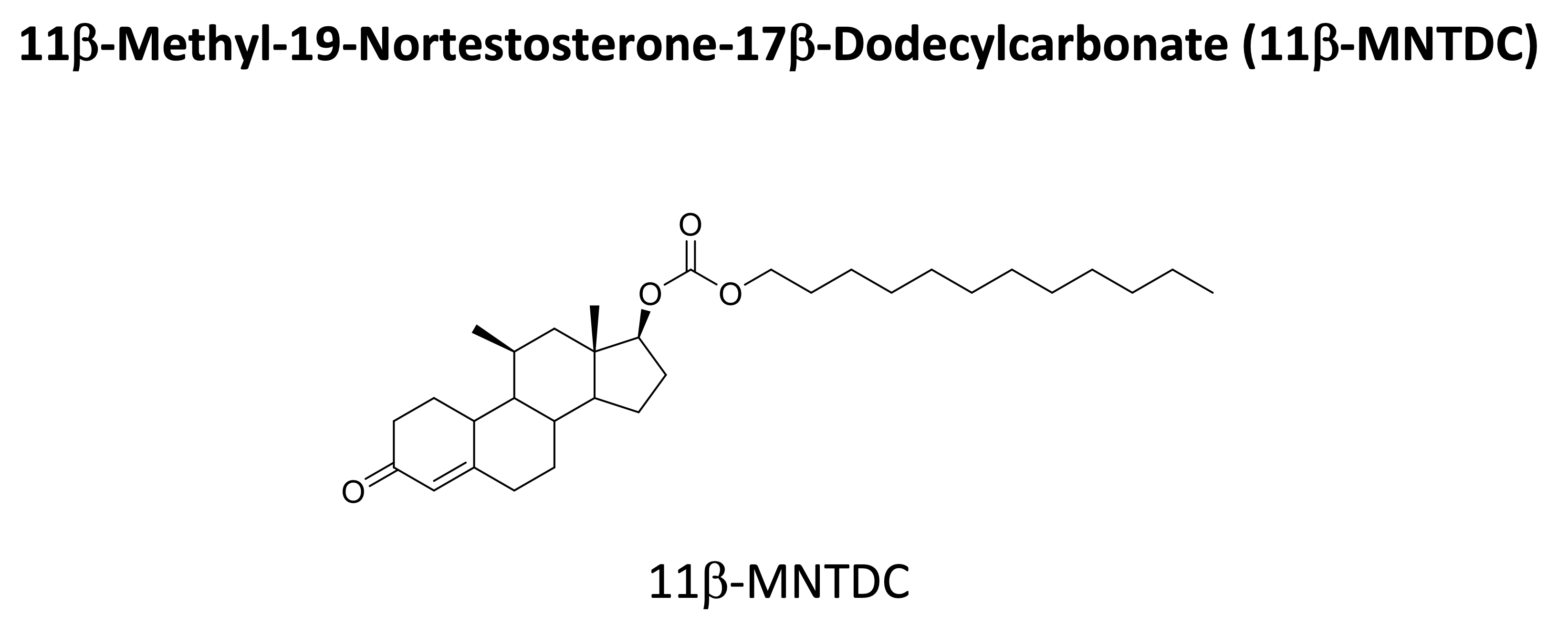
Since 2016, NICHD has been testing the following lead compound as a male contraceptive in Phase I clinical trials:
Safety and pharmacokinetics of oral single-dose, 28 day repeat-dose, and dose escalation study of 11-MNTDC in healthy men have been conducted. The drug was well tolerated without serious adverse events. Daily oral dose of 11β-MNTDC for 28 days in healthy men showed markedly suppressed serum gonadotropins and T concentrations without serious adverse effects. NICHD is planning another Phase I trial to test 11β-MNTDC via intramuscular injection.
Researchers at the Eunice Kennedy Shriver National Institute of Child Health and Human Development are highly motivated in seeking licensing and/or collaboration partners for the development and use of androgenic compounds as contraceptives and/or hormonal therapeutics.
Competitive Advantages:
- Significant market need for male contraceptive
- Clinical-stage asset
- No adverse events in a Phase I dose-escalation study
- Intramuscular (IM) injection
Commercial Applications:
- Male contraceptive
- Treatment of hormonal diseases
Licensing Contacts
Patents
- US
Provisional (PRV) 60/650,376
Filed on 2005-02-04
Status: Abandoned - Patent Cooperation Treaty
(PCT) PCT/US2006/02436
Filed on 2006-01-24
Status: Expired - Australia
National Stage 2006211907
Filed on 2006-01-24
Status: Abandoned - Canada
National Stage 2596884
Filed on 2006-01-24
Status: Abandoned - European Patent
National Stage 06719336.7
Filed on 2006-01-24
Status: Abandoned - Japan
National Stage 2007-554134
Filed on 2006-01-24
Status: Abandoned - US Patent 7,820,642
Filed on 2007-08-03
Status: Issued - Germany
European patent (EP) 06719336.7
Filed on 2006-01-24
Status: Abandoned - France
European patent (EP) 06719336.7
Filed on 2006-01-24
Status: Abandoned - United Kingdom
European patent (EP) 06719336.7
Filed on 2006-01-24
Status: Abandoned
Publications
- Nguyen, BT, et al. Acceptability of the oral hormonal male contraceptive prototype, 11β-methyl-19-nortestosterone dodecylcarbonate (11β-MNTDC), in a 28-day placebo-controlled trial
- Wu, S, et al. Safety and Pharmacokinetics of Single-Dose Novel Oral Androgen 11β-Methyl-19-Nortestosterone-17β-Dodecylcarbonate in Men.
- Yuen, F, et al. Daily Oral Administration of the Novel Androgen 11β-MNTDC Markedly Suppresses Serum Gonadotropins in Healthy Men.
Collaborations
- Licensing
- Collaboration
Date Published
